2.6 mio. DKR. granted by Danmarks Frie Forskningsråd (Independent Research Fund Denmark) to Hans Ulrik Riisgård, together with Jonathan Brewer (SDU) and Peter Funch (AaU) for 2 years (2018-2020).
Abstract: Sponges were present more than 600 million years ago and have ever since been important components in aquatic ecosystems. They are sessile and pump huge amounts of water through their bodies from which they filter out food particles. Most likely they share basic characteristics with more advanced filter-feeders that all possess protection and cleaning mechanisms to overcome particle-overloading. The goal of the project is to understand the mechanisms ensuring maintenance of the sponge filter-pump by providing a basic understanding of the origin and purpose of contractile behaviour of sponges. Understanding of the ecophysiology of sponges and comparison with other filter-feeders, such as the blue mussel that reduces its respiration rate to overcome starvation during winter months, may shed light on the evolutionary and ecological success of sponges – the world’s simplest multicellular animals.
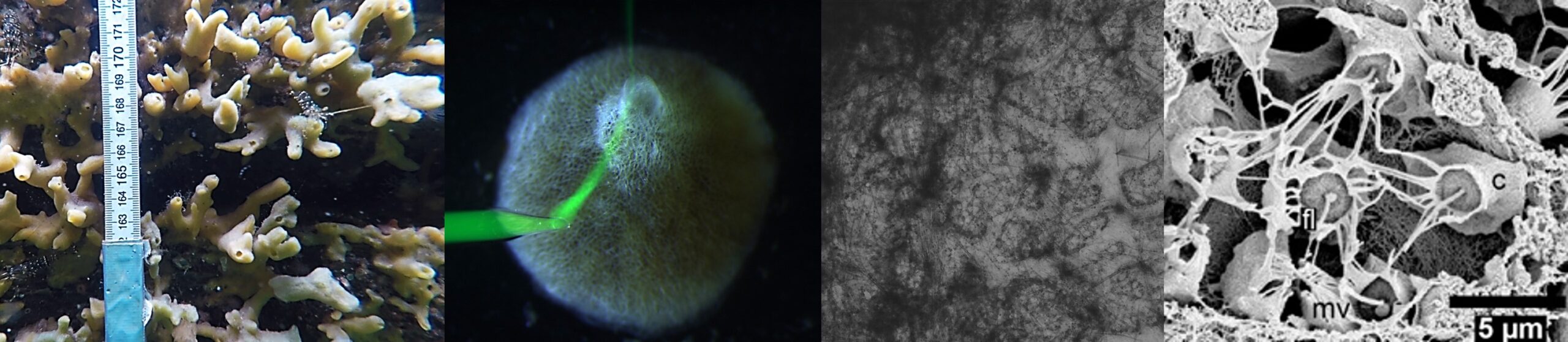
Halichondria panicea. Single-osculum sponge explant after exposure to 100 mg L−1 (i.e., ∼106 particles mL−1) inorganic marl particles (size range 2 to 25 µm) for 2 h and subsequent transfer to particle-free seawater at time t = 0. Water-pumping activity is evident from clumps of inorganic particles entangled in mucus frequently expelled through the osculum with the exhalant jet. White particles accumulate on the sponge surface during the video-observation time (duration: 16 s, frame rate: 60.61 fps). From: Goldstein et al. (2024)

Contractile phases of a single-osculum sponge explant (Halichondria panicea); side-view projected area and osculum (arrows) are visible. I: Phase of contraction with osculum closure and reduction in projected area; II: contracted phase with closed osculum and minimum projected area; III: phase of expansion with opening of osculum and increase in projected area; IV: expanded phase with open osculum and maximum projected area. Scale bar: 1 mm. From: Goldstein et al. (2020)
Time-lapse observation (1-min-intervals; 24 fps) of an undisturbed Halichondria panicea sponge explant (top view). The sponge shows spontaneous contractions that involve its entire aquiferous system, including in-/excurrent canals and the osculum. Scale bar: 1 mm. From: Goldstein et al. (2019)
Publications
Goldstein, J., Bisbo, N. Funch, P., Riisgård, H.U. (2019). Contraction-expansion and morphological changes of the aquiferous system in the demosponge Halichondria panicea. Front. Mar. Sci. – Aquatic Physiology 7: 113. https://doi.org/10.3389/fmars.2020.00113
Larsen, P.S., Riisgård, H.U. (2021). Pumping rate and size of demosponges – towards an understanding using modeling. J. Mar. Sci. Eng. 9: 1308. https://doi.org/10.3390/jmse9111308
Riisgård H.U., Larsen, P.S. (2022). Actual and model-predicted growth of sponges – with a bioenergetic comparison to other filter-feeders. J. Mar. Sci. Eng. 10: 607. https://doi.org/10.3390/jmse10050607
Riisgård, H.U., Larsen, P.S. (2022) Filtration rates and scaling in demosponges. J. Mar. Sci. Eng. 10: 643. https://doi.org/10.3390/jmse10050643
Larsen, P.S., Riisgård, H.U. (2022). Seize-specific growth of filter-feeding marine invertebrates. J. Mar. Sci. Eng. 10: 1226. https://doi.org/10.3390/jmse10091226
Funch, P., Kealy, R.A, Goldstein, J., Brewer, J.R., Solovyeva, V., Riisgård, H.U. (2023).Fate of microplastic captured in the marine demosponge Halichondria panicea. Mar. Pollut. Bull. 194: 115403.
Riisgård, H.U., Kealy, R.A, Goldstein J., Brewer J., Solovyeva V., Funch, P. (2023). Choanocyte dimensions and pumping rates in the demosponge Halichondria panicea. J. Exp. Mar. Biol. Ecol. 569: 151957. https://doi.org/10.1016/j.jembe.2023.151957
Riisgård, H.U. (2024). Oxygen extraction efficiency and tolerance to hypoxia in sponges. J. Mar. Sci. Eng. 12(1): 138. https://doi.org/10.3390/jmse12010138
Riisgård, H.U., Lüskow, F., Larsen, P.S. (2024). Growth, filtration, and respiration characteristics of small single-osculum demosponge Halichondria panicea explants. J. Exp. Biol. 227: jeb247132. https://doi.org/10.1242/jeb.247132
Goldstein, J., Riisgård, H.U., Kealy, R.A., Funch P. (2024). Particle loads, contractile responses and cleaning in the demosponge Halichondria panicea. J. Exp. Mar. Biol. Ecol. 577: 152021.
Lees, M.K.,Riisgård, H.U., Larsen, P.S. (2024) Flow pattern, particle capture, and contractile behaviour in the demosponge Halichondria panicea. Mar. Biol. Res. 1-10. https://doi.org/10.1080/17451000.2024.2390527
Riisgård, H.U., Larsen P.S. (2024). Energy costs of the sponge pump. Mar. Ecol. Prog. Ser. 746: 141-151.
Goldstein, J., Bisbo, N., Funch, P., Daugaard, N.D., Larsen, P.S., Brewer, J.R., Riisgård, H.U. (2024). Aquiferous system, filtration rates and hydrodynamics of a syconoid calcareous sponge Urna sp. Mar. Biol. 171: 216. https://doi.org/10.1007/s00227-024-04532-0
Kumala, L., Riisgård, H.U. (2024). Growth, filtration and respiration under superfluous feeding in single-osculum single-osculum Halichondria panicea sponges. Oceans (accepted after changes)